Call for Validation of biomarker candidates 2025

Biomarkers, as measured by (molecular) imaging, in blood, urine, stool or breath, are key to identify patients at risk of developing cancer, to predict/monitor treatment responses and to detect recurrences.
Despite decades of discovery-driven biomarker studies, only a small number of biomarkers have been successfully validated and implemented in daily care. A reason is that translation and implementation of the biomarkers requires multidisciplinary teams that might differ in composition and expertise from the team making the initial biomarker discovery. Moreover, clinical decision-making often is based on the input of several biomarkers, again requiring a team with complementing expertise. Finally, it takes a lot of work and a variety of both laboratory and applied clinical studies to set the stage for widespread clinical acceptance of new biomarkers.
Ambition
To clinically validate (combinations of) already known biomarkers that align with patient relevant outcomes or treatment responses and that can be used in daily clinical practice in the near future (TRL5/6). To achieve this, efforts are needed to set up collaborative, multidisciplinary studies with special attention to clinical translation. The focus of this call is to bring together the expertise in the field of biomarker research, the clinical care of end users and the needs of patients. By focusing on implementation rather than discovery, we strive to provide the appropriate framework and funding to realize the implementation of valuable biomarker candidate(s).
Definition
Biomarkers: (Bio)characteristics that are objectively measured and evaluated as an indicator for cancer risk, the presence of a specific cancer or recurrent cancer, the stage or aggressiveness of a specific cancer, and how well the patient responds to therapeutic intervention(s).
Requirements
- Research type: (multidisciplinary) consortium addressing the required variety of disciplines with a minimum of 4 participating parties (which may include private partners)
- Research phase: preclinical/clinical.
- Cancer type: all cancer types.
- Known biomarkers that are not yet clinically validated/accepted but that provide an added value in clinical care (TRL5/6).
- Multidisciplinary team supported by a project manager.
- Participating parties: all required expertise, including e.g., biostatistician, HTA-expert (in-house service/not-for-profit organization). Public/Private collaborations are encouraged if co-funding is in place. KWF holds the right to request additional information about the for-profit partner if this is deemed necessary.
- Sustainable data sharing plan (according to FAIR principles).
- Detailed developmental plan; including the consequences for patient outcomes and health care costs after potential implementation.
- An (early)HTA is a mandatory component of the application.
- The project addresses patient needs; including a description of the patient journey.
- The perspective of patients is incorporated. Patients/patient associations are closely involved during the set-up of the proposal, the conduction of the research, and the sharing of the (lay)results with the target group.
In case you have a valid reason, you may deviate from the eligibility condition. This valid reason must be substantiated in the application.
In Scope
Validation of already known biomarkers in the relevant environment that align with patient relevant outcomes or treatment prediction/ responses and that can be used in daily clinical practice to improve oncological outcomes in the near future (TRL5/6).
Out of scope
Biomarkers to identify/predict side effects of cancer treatment are not included.
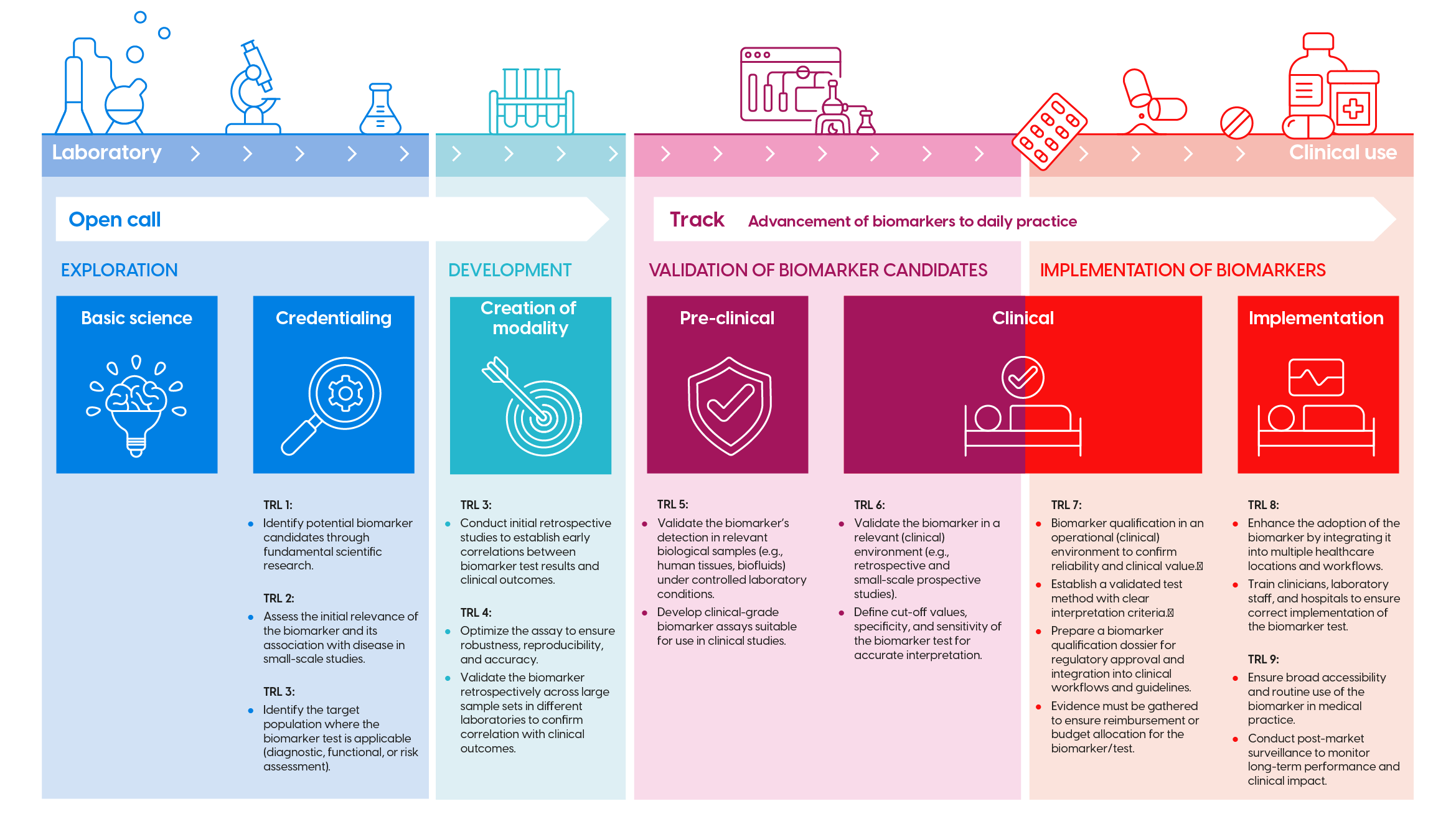
Biomarker development trajectory
The development of biomarkers consists of successive research phases and Technology Readiness Levels (TRL). These are visualized in the infographic above (click to enlarge). For each phase, KWF has corresponding funding opportunities (open calls, theme call, etc.). Check the infographic to determine if your proposal fits the appropriate call.
Terms & conditions
The KWF Funding Conditions 2025 and the KWF Accountants Protocol 2025 apply (see downloads).
Specific guidelines on the process, characteristics and eligibility terms for the Thematic Call 2025 will be based on the current KWF Guidelines. Guidelines will include specific information on application requirements, preferences and recommendations, review procedure and timelines, and estimated total budget. Granted KWF projects will be funded under the current Funding Terms and Conditions.
Additional conditions (in addition to the standard KWF funding conditions)
- If the for-profit private partner, larger >250 FTE, has an active role within the project, this party is considered a participating party and to this end must make a financial contribution: this is 10% co-financing of the KWF requested budget (in kind and/or in cash).
- There is a maximum hourly rate for payroll costs:
- Supportive (MBO) € 85,00
- Project Management (HBO) € 100,00
- Expert (WO) € 125,00
This is the maximum hourly rate excluding VAT and including all other costs (travel costs, parking costs, travel hours, etc.) as they should be applied. If institutes would like to apply a higher hourly rate for motivational reasons, this must always be agreed and approved in advance by KWF.
Additional project specific conditions may be applied.
Timeline
| Opening pre-proposals: | 4 March 2025 |
| Closure pre-proposals: | 29 April 2025 (12.00 noon) |
| Opening full proposals: | 17 June 2025 |
| Closure full proposals: | 9 September 2025 (12.00 noon) |
| Interviews: | November 2025 |
| Funding decision: | December 2025 |
Recommendations and considerations for applicants and reviewers
The Dutch Cancer Society includes a pre-proposal round for this call to be able provide recommendations for the researchers and input and/or guidance for the reviewing committee. Those additional considerations for proposals, which allow prioritization if necessary are:
- A proven regulatory/market-access strategy and/or regulatory support
- Make use of or participate in existing central facilities and (data) infrastructures (databases, biobanks, imaging data, etc.)
- If not yet participating, an advisory board with parties that are familiar with the implementation of a biomarker in daily practice is a pre.
Indicative budget and duration
Total budget: 4-6M
Budget per proposal: 0.8-2M
Duration: 2-5 years
Evaluation
KWF uses three review criteria: relevance for KWF’s main goals, scientific quality and feasibility. In addition, the emphasis of this thematic call will be on moving the findings forward and the final patient’s health gain and not so much on novelty of the findings, which is in line with the advancement to daily practice. A special review committee will be selected for this call, consisting of experts in relevant areas. Additionally, the full proposal will also be reviewed by the patient advocacy committee (PACO).
After evaluation of the full proposals the (multidisciplinary) consortia will be invited for an interview.